Just as the course of a drift boat can be irreversibly altered by a log in its path, a single mutation can send life in an entirely new direction.
That scenario, says UO biochemist Ken Prehoda, provides a window on how one mutation sparked a huge jump in the evolutionary course of a protein important for the evolution of animals.
Earlier this year, Prehoda was on a team that found that a random mutation 600 million years ago in a single-celled organism created a new family of proteins that are important for multicellular life. In a new paper, now online ahead of print in the Journal of the American Chemical Society, Prehoda and colleagues describe what the mutation did to the original protein family.
RELATED LINKS
Mutations happen randomly. Most are bad news. But occasionally a mutation is good, helping an organism adapt to environmental changes or advancing overall fitness. Understanding such changes better, Prehoda said, could potentially point to new treatments for human diseases such as cancer.
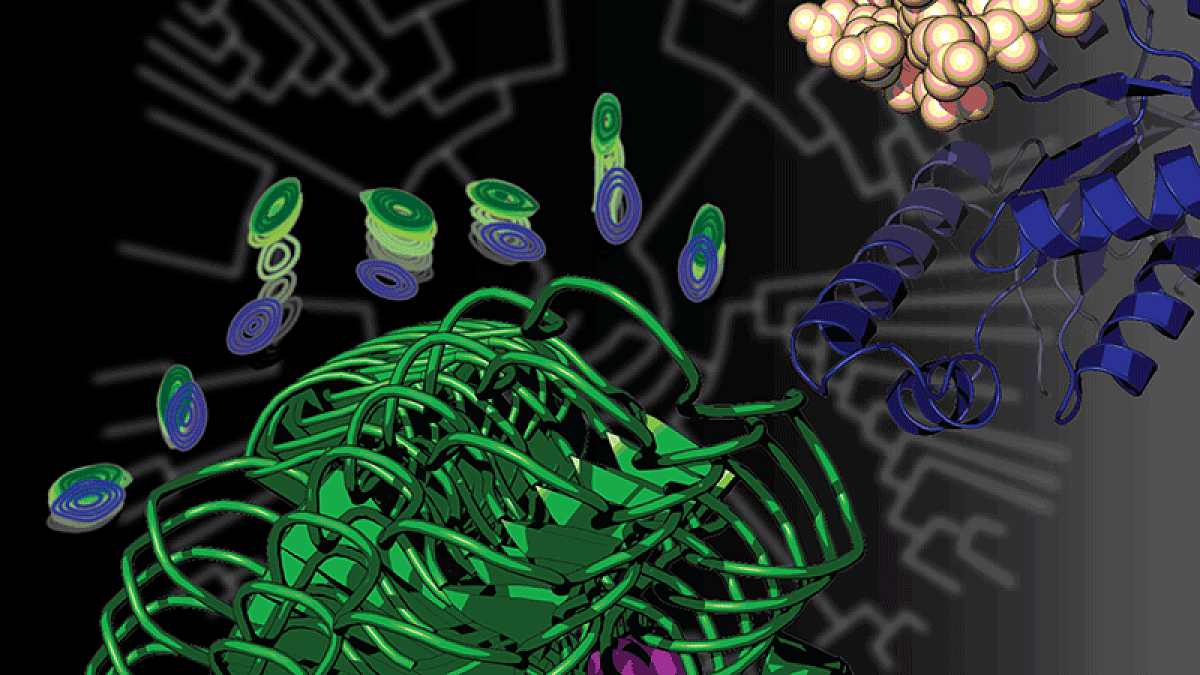
Prehoda's lab initially used a molecular technique called ancestral protein reconstruction. The technique allows researchers to move backward in the evolutionary tree to see molecular changes and infer how proteins performed in the past.
For the new study, Prehoda’s lab collaborated with researchers at the Medical College of Wisconsin who studied whether the mutation changed the flexibility of the protein. Next, his team turned to computer simulations in the UO's High Performance Computing Research Core Facility to explore how altered flexibility led to changes in the protein’s interactions.
"We found that this mutation that helped our unicellular ancestor to become multicellular, and ended up leading to an entirely new family of proteins that are specific to animals, did so in a very interesting way," Prehoda said. “Amazingly, this one mutation took a protein that was really flexible — an important trait for its old job — and made it much more rigid so it could advance to a new function."
The mutation, which researchers labeled s36P, set off a cascade of events in which protein interactions took new routes and evolved into more complex multicellular organisms, Prehoda said. The mutation is still conserved in all animals today, he added.
"A lot of the proteins that do the work in our bodies can be thought of as molecular machines," Prehoda said. "They move in a way that is coordinated with function. Each protein spins in a circle or motors along filaments. Our protein, before the mutation, was an enzyme that had certain flexible movements related to its function. This one mutation fixed the protein’s backbone, locking the molecule into a shape that is important for its new function."
Prehoda and colleagues reported their discovery of mutation in a paper that appeared Jan. 7 in the journal eLife.
The new paper will be highlighted on the cover of an upcoming print edition of the Journal of the American Chemical Society. Co-authors are Dustin S. Whitney and Brian F. Volkman, both of the Medical College of Wisconsin in Milwaukee. They also were among the co-authors on the eLife paper.
—By Jim Barlow, University Communications