In science, persistence can pay off.
A long-running mystery involving a mutant protein found 17 years ago in the UO biology lab of Bruce Bowerman has been solved, with details appearing in a paper placed online by the journal eLife earlier this month.
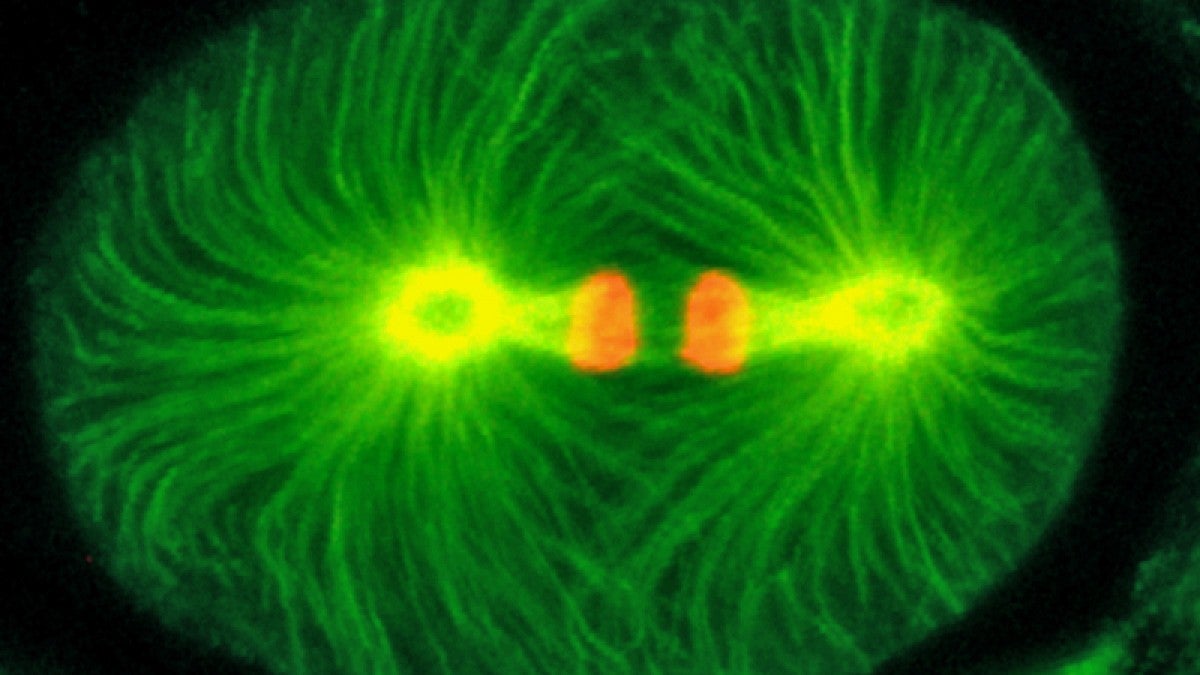
In the world of basic research, the findings are a big deal. The protein — pulled from roundworms, a classic model organism in biology — is now considered the earliest known actor in the early cell division that is vital to the molecular machinery that directs the genetic traffic that builds all animals.
More details about the scientific significance of the eLife paper can be seen in a separate UO news release.
The findings, said Bowerman, who now heads the Department of Biology, enhance the basic understanding of cell division. Whether human medical treatments may be realized is difficult to say, but the cellular machinery involved in roundworms is similar across the animal kingdom, he said.
"The biggest advances in biomedical research that lead to dramatic changes in human health are usually the product of basic science that was never intended to lead to a biomedical advance," he said. "Antibiotics and early cancer treatments emerged from basic research."

"I looked at it just enough to think it looked cool and would make a nice project for me to take away to work on when I started my own faculty position and lab," said Hamill, who left the UO in 2001 and landed at Ohio Wesleyan University, a small undergraduate-based liberal arts college in a small town north of Columbus.
Hamill and Bowerman kept looking — Bowerman in his lab, and Hamill with undergraduate students in her lab.
With the arrival of whole-genome sequencing technology at the UO, Hamill returned to campus in 2008 and 2014 for short sabbaticals to explore the mutant. Working with former UO graduate student Joshua Lowry, she put a label on the molecule. A new postdoctoral researcher, Kenji Sugioka, in Bowerman's lab soon joined the team.
At that point, however, the protein's function remained unknown, Sugioka said, but that changed when Bowerman sought help from his former postdoctoral mentor at the University of Washington, James R. Priess, now also a researcher at the Fred Hutchinson Cancer Research Center in Seattle and an expert in high-resolution transmission electron microscopy.
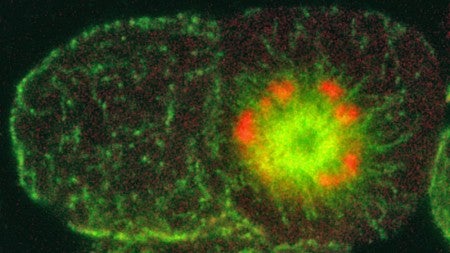
Working with Priess, Sugioka obtained images of roundworm embryos that had been sliced into hundreds of sections. Turning next to a sophisticated technique known as laser-scanning confocal microscopy at the UO, Sugioka grasped the significance of the mutant protein and helped identify a vital structural component in the cell-division machinery that has been observed but not understood in other animals.
"We expect that a sas-7-like protein is working in human cells, but it has not yet been identified," Sugioka said. "I hope our contributions lead to the understanding of these processes."
"I've worked on this, on and off, for more than 15 years with my students," said Hamill, a professor of zoology. "Sometimes things went well and we made good progress, and then we'd hit snags and get hung up. Standard steps that worked for characterizing similar mutants often just didn't work in this case. There were several times I felt like we should throw in the towel on this project, but it seemed unique and interesting, so we kept trying different approaches."
Being a part of the longest-running project in Bowerman's lab — funded primarily by the National Institutes of Health — was a great experience, said Sugioka, who was supported by a Human Frontier Science Program grant from the France-based International Human Frontier Science Program Organization and a Journal of Cell Science travel fellowship.
"This project has been very gratifying," said Bowerman, a member and former director of the Institute of Molecular Biology. "It's an ultimate example of how persistence pays off."
—By Jim Barlow, University Communications