Bleach kills germs, so most people would assume that it would be a repellent to bacteria. But new research from the lab of UO biologist Karen Guillemin shows how one common bacterium linked to stomach cancer is attracted to bleach.
“We found that these bacteria can swim toward sources of bleach and that it can actually tolerate pretty high concentrations and not be killed,” said Arden Perkins, a postdoctoral fellow and the lead author on a new paper in PLOS Biology investigating the bacterial pathogen Helicobacter pylori and how it senses bleach at the atomic level.

The connection to bleach is important because white blood cells in the human body actually produce and secrete bleach to ward off infection.
“The same chemical compound that you would use to disinfect a countertop, at the molecular level, our immune cells are able to produce that compound,” Perkins said.
But, he said, these bacteria have figured out a way to be very successful in the presence of bleach and in the human stomach, a harsh environment that most bacteria do not inhabit. In general, Perkins said, bleach is still effective at killing bacteria, but for short periods of time the H. pylori bacteria are able to survive exposure to bleach and can survive much higher concentrations of bleach than expected.
To better understand how and why the bleach-sensing mechanism functioned, Perkins used his expertise in mapping the three-dimensional structures and chemistry of proteins to reveal the processes at work at a molecular and atomic level.
Researchers found that the bacteria relied upon a particular protein known as TlpD to sense bleach. Exposure to bleach stimulates the TlpD protein to send an attractant signal to the bacteria’s flagella — threadlike tails that help bacteria move — that causes the bacteria to swim toward sources of bleach.
The research team believes that the H. pylori bacteria may be attracted to bleach as part of a strategy to remain at sites of inflammation long term. Bleach is a hallmark of tissue inflammation, and the H. pylori bacteria make a living of promoting and inhabiting inflamed tissue for years or even decades.
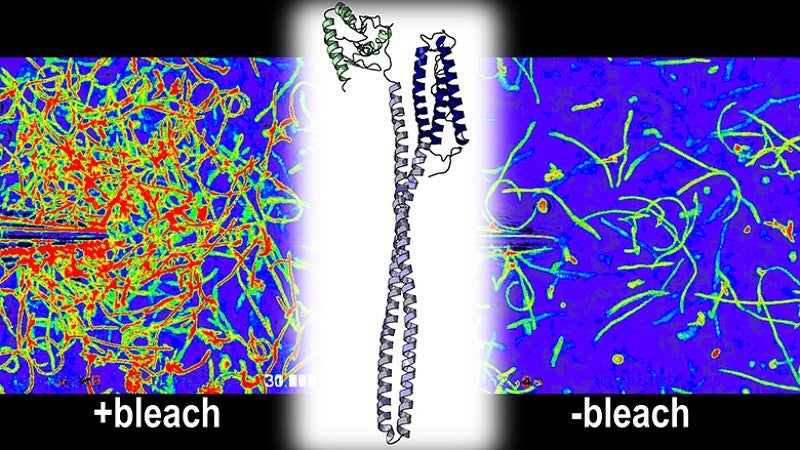
Researchers found that the bacteria salmonella and E. coli also have TlpD-like proteins that can detect bleach, suggesting this is a previously unrecognized strategy bacteria use to sense host inflammation.
“That’s a very important scientific contribution, that we understand the machinery of bleach sensing,” Guillemin said. “It turns out that this is not a machinery that is exclusive to Helicobacter pylori and allows us insights into other bacteria that have similar machines.”
The research could eventually lead to new therapies to disrupt the bleach-sensing function of harmful bacteria and could have implications for reducing antibiotic resistance. Typical antibiotics that are used clinically today kill or prevent bacteria from dividing by targeting things like the bacterial cell wall. As a result, bacteria face selective pressure to develop resistance to those kinds of drugs in order to survive.
In the case of H. pylori, 30 percent of clinical isolates are now resistant to antibacterial treatment. With a more thorough understanding of the mechanisms at work, Guillemin said, researchers may be able to develop new, more effective means of combating bacteria.
“It might be that there are less strong selective pressures for bacteria to overcome a drug that just makes them disoriented,” Guillemin said. “By 2050 there’s going to be pandemics of antibiotic resistant bacteria so there’s a real need to think about new strategies to disrupt bacteria.”
Researchers Jim Remington, professor emeritus in the Department of Physics, and Dan Tudorica, an undergraduate biochemistry major at the UO, contributed to the study along with Manuel Amieva, professor of pediatrics and microbiology and immunology at Stanford University School of Medicine. The research was supported by the National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Disease under award numbers R01DK101314 5 and F32DK115195.
—By Lewis Taylor, University Communications