Teamwork by chemists in two University of Oregon labs has led to a new class of fluorescent dyes that could expand the real-time view of cell activity in medical diagnostics.
Conversations and experiments involving a doctoral student, a postdoctoral researcher and three chemistry professors paved the way for the discovery, which took three years and is now headed into testing for possible use in medical imaging in a joint project with Oregon Health & Science University.
“This effort involved pulling together multiple ideas with multiple people,” said doctoral student Brittany White. “Having such a great environment for collaboration between labs is really what made this project possible.”

In a paper in ACS Central Science, with White as lead author, the group detailed how they fitted organic molecules called nanohoops with a chemical sidechain of sulfonate to make them water soluble and able to penetrate cell membranes. Nanohoops are made with short, circular slices of carbon atoms. Making them in different sizes, the group discovered, produces distinctive colors that can be illuminated in living cells by a single laser burst.
Thousands of chemical dyes are used in biological studies, but typically only three or four often-overlapping colors can be visible in experiments, said study co-author Bruce Branchaud, professor emeritus at the UO and a distinguished scientist in the Cancer Early Detection Advanced Research Center of OHSU’s Knight Cancer Institute. He once headed dye chemistry for Molecular Probes/Invitrogen, a world-leading fluorescence biotechnology company located in Eugene.
“The key thing about this work is that the majority of dyes used for any purpose in the last 5,000 years have been flat molecules,” he said “Because of the novel nonplanar, circular structures of nanohoops, they have unique and powerful properties that potentially will give them a lot of value in the marketplace of biomedical sciences.”
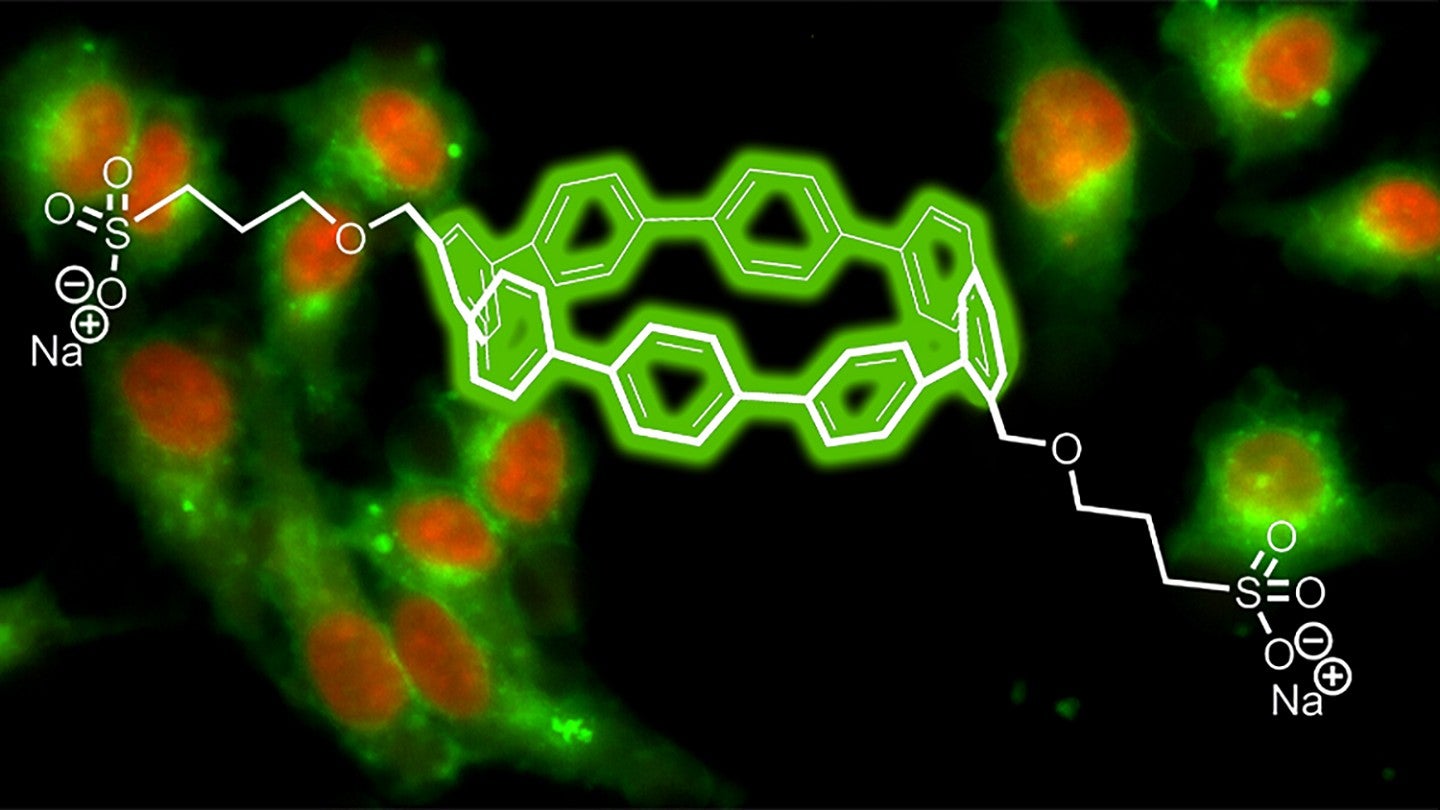
In 2008, Ramesh Jasti, then a postdoctoral fellow at the Lawrence Berkeley National Laboratory, was the first scientist to synthesize nanohoops. Now a UO chemistry professor, he has continued to pursue them for use in electronic and optical devices, solar cells, light-emitting diodes and medical sensing.
RELATED LINKS
The idea of using nanohoops as dyes emerged from discussions between Branchaud and members of Jasti’s and fellow chemistry professor Mike Pluth’s labs. White, based in Jasti’s lab, was paired with postdoctoral researcher Yu Zhao of Pluth’s lab to find a way to make the nanohoops soluble. Taryn Kawashima, winner of a 2016 undergraduate fellowship award by the Office of the Vice President for Research and Innovation, helped in the experiments and was a co-author on the study.
White, a native of Manchester, New Hampshire, had enrolled in graduate school at Boston University to study under Jasti, just before he was offered a position at the UO. It was a no-brainer, she said, to follow him to Eugene, where he had promised that a collaborative atmosphere awaited them. She gladly became part of the Graduate School’s population of 3,200 graduate students, 51 percent of whom are women.
“I love chemistry,” White said. “I love making interesting molecules. That’s what I had in my head when I entered graduate school. This project, though, opened my eyes to the field of chemical biology, and I realized how interested I am making different unique things that can be used for human health or have some kind of downstream effect in biology. This project really gave me the exposure that let me see how chemistry, biology and physics can all tie together.”
Zhao taught White how to incubate human cells for use with the nanohoops. White was a fast learner, said Zhao, who joined the Pluth lab in 2016 after earning a doctorate at Washington State University and spending a year as a research associate at the Scripps Research Institute in Florida.
“I’ve learned so much,” White said. “Just being able to go to Mike Pluth’s and Bruce Branchaud’s offices and learn from Yu has let me take everything to the next level. This type of collaborative environment makes the Department of Chemistry and Biochemistry unique.
“I remember that day in the Pluth lab when, holding our breaths, Yu and I went up to the microscope and the image of a fluorescent molecule we were hoping to see came up,” she said. “We were like, ‘Yes! Yes! We finally got it!’ I took a picture and texted it to Ramesh.”
The project also has given career direction to White. She leaves the UO at year’s end to begin a postdoctoral research position in chemical biology at Cornell University.
The collaboration, Zhao said, also has been a bonus in his professional training.
“The goal of my career is to apply chemical tools that I will develop to understand the biology of small molecules in living systems,” he said. “The teamwork spirit I found in this project will significantly facilitate my interdisciplinary research.”
Jasti is now working with Xiaolin Nan of the Department of Biomedical Engineering at OHSU to pursue the use of the nanohoops in biological imaging. The project is among 10 funded under the 2018 OHSU-UO Collaborative Seed Grant program.
—By Jim Barlow, University Communications

