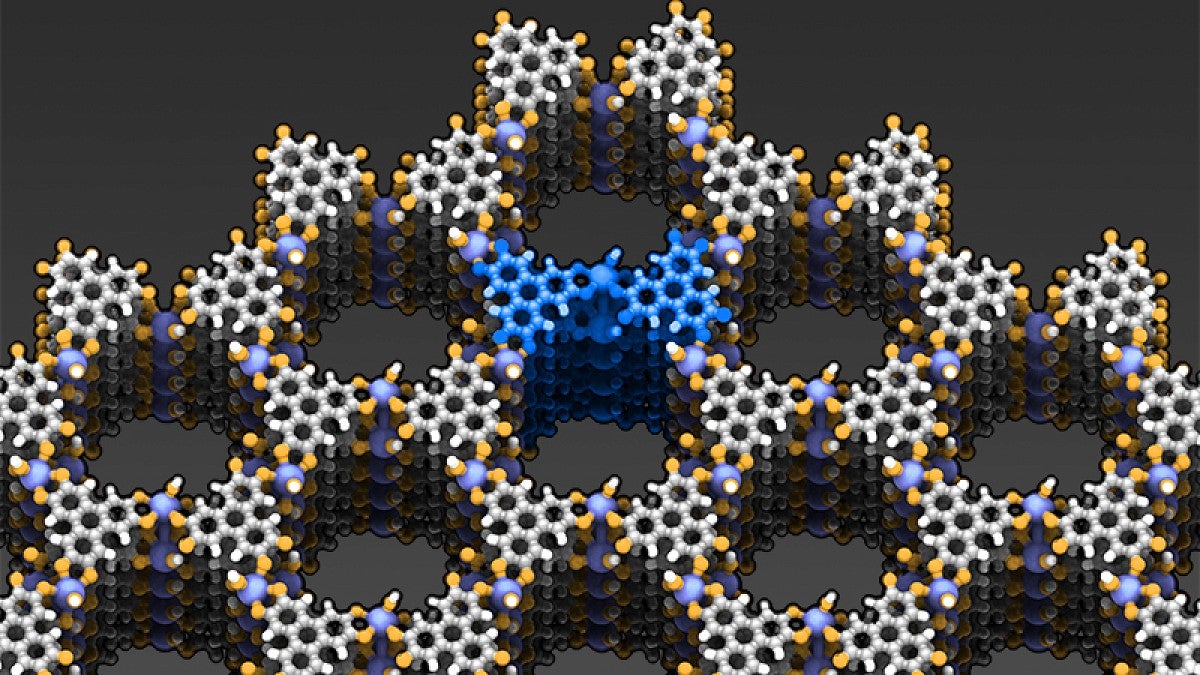
University of Oregon chemists analyzing a new porous material synthesized by a research group at the Massachusetts Institute of Technology have discovered that electrical charges flow through it in an unexpected but potentially advantageous way.
The material acts as metal in one direction and a semiconductor in other directions, properties that allow the electrical charges to flow between atoms, a six-member research team reported in the journal Nature Chemistry.
Such materials eventually could lead to new-age supercapacitors and batteries that deliver fast and precise pulsed power, said Christopher Hendon, a professor in the UO’s Department of Chemistry and Biochemistry and member of the Materials Science Institute.
“This is an important result, because it means that charges are flowing through material in a direction where things are not technically touching,” Hendon said. “As a design principle, doing that is something we’ve been working on a long time.”
The collaborative research builds on efforts that began 20 years ago by numerous labs to produce electrically conductive metal organic frameworks, sponge-like material with extremely high surface areas. If a couple of grams of the material were flattened, Hendon said, the material would cover a space about the size of the UO’s Autzen Stadium.
Hendon had worked on early modeling of such frameworks during his doctoral research at the University of Bath in the United Kingdom. That work continued during his postdoctoral work at MIT, and he brought his related theoretical research to the UO when he arrived in 2017 as a member of the Energy and Sustainable Materials Initiative.
“We’ve taken a type of material that we have conventionally thought of as simply a sponge and demonstrated that you can conduct electricity through it in different directions,” he said. “As a result, these types of materials now have broadened their applications to energy storage devices.”
Research on materials using a variety of other charged elements is rapidly evolving. Material similar to that analyzed in the paper is part a new joint MIT-Lamborghini project to produce supercapacitors for use in the automaker’s efforts to build an electric supercar. Another automaker, Tesla, earlier this year acquired Maxwell, a manufacturer of ultracapacitors.
The material studied in Hendon’s theory-driven materials simulation lab at the UO was made by Grigori Skorupskii in Mircea Dinca’s MIT lab. It was produced by mixing an organic molecule known as hexahydroxytriphenylene with a series of lanthanide elements – lanthanum, neodymium, holmium and ytterbium – in a liquid solvent and water.
After the paper’s co-authors, Benjamin A. Trump and Craig M. Brown, determined the structural makeup of the crystals in the material, Hendon and his UO graduate student, Thomas W. Kasel, who has since earned his master’s degree, studied the electrical activity. Trump and Brown are both at the National Institute of Standards and Technology in Gaithersburg, Maryland.
A challenge in making conductive metal organic frameworks, Hendon said, is that charges traditionally are expected to flow in the direction of the points of connectivity.
“In this case, however, we noticed that the material was conducting and suspected that the current was flowing in the nonconnected direction,” he said. “Our theory led us to find that the conductivity was flowing perpendicular to the connectivity.”
That, he added, means that conductivity can be driven by how stacked two-dimensional sheets are fitted above and below the new spongy organic-lanthanide material.
“This paper lays a foundation that says it is possible to make a tunable electrical conductor that does not feature physical bonds in the direction of conductivity,” Hendon said. “There has been a lot of work where researchers have been hypothesizing about molecules stacking and conducting. In this case, what we observed has been highly evasive.”
The research, he said, could lead to devices that rapidly charge and discharge pulses of high voltage without generating high current and associated heat. That could be a supercapacitor that would charge as a car brakes and then discharge during rapid acceleration, or a sunlight-powered jet pack like the one used in the first “Iron Man” movie.
The U.S. Army Research Office (grant W911NF-17-1-0174) and National Science Foundation (grant 1053575) primarily supported the project.
—By Jim Barlow, University Communications